The Transderm Scop (transdermal
scopolamine) system is a
circular flat patch designed for continuous release of scopolamine
following application to an area of intact skin on the head, behind the
ear. Each system contains 1.5 mg of scopolamine base. Scopolamine is
alpha-(hydroxymethyl) benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo
[3.3.1.02,4] non-7-yl ester. The empirical formula is C17H21NO4
and its structural formula is
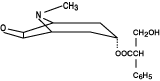
Scopolamine is a viscous liquid that has a molecular weight of 303.35 and a pKa of 7.55-7.81. The Transderm Scop system is a film 0.2 mm thick and 2.5 cm2, with four layers. Proceeding from the visible surface towards the surface attached to the skin, these layers are: (1) a backing layer of tan-colored, aluminized, polyester film; (2) a drug reservoir of scopolamine, light mineral oil, and polyisobutylene; (3) a microporous polypropylene membrane that controls the rate of delivery of scopolamine from the system to the skin surface; and (4) an adhesive formulation of light mineral oil, polyisobutylene, and scopolamine. A protective peel strip of siliconized polyester, which covers the adhesive layer, is removed before the system is used. The inactive components, light mineral oil (12.4mg) and polyisobutylene (11.4mg), are not released from the system.
Cross section of the system:
| Backing Layer |
| Drug Reservoir |
| Rate-Controlling Membrane |
| Contact Adhesive |
| Protective Peel Strip |
Pharmacology
The sole active agent of Transderm Scop is scopolamine, a belladonna alkaloid with well-known pharmacological properties. It is an anti-cholinergic agent which acts: i) as a competitive inhibitor at postganglioinic muscarinic receptor sites of the parasympathetic nervous system, and ii) on smooth muscles that respond to acetylcholine but lack cholinergic innervation. It has been suggested that scopolamine acts in the central nervous system (CNS) by blocking cholinergic transmission from the vestibular nuclei to higher centers in the CNS and from the reticular formation to the vomiting center1,2. Scopolamine can inhibit the secretion of saliva and sweat, decrease gastrointestinal secretions and motility, cause drowsiness, dilate the pupils, increase heart rate, and depress motor funtion2.
Pharmacokinetics
Scopolamine's activity is due to the parent drug. The pharmacokinetics of scopolamine delivered via the system are due to the characteristics of both the drug and dosage form. The system is programmed to deliver in-vivo approximately 1.0mg of scopolamine at an approximately constant rate to the systemic circulation over 3 days. Upon application to the post-auricular skin, an initial priming dose of scopolamine is released from the adhesive layer to saturate skin binding sites. The subsequent delivery of scopolamine to the blood is determined by the rate controlling membrane and is designed to produce stable plasma levels in a therapeutic range. Following removal of the used system, there is some degree of continued systemic absorption of scopolamine bound in the skin layers.
Absorption: Scopolamine is well-absorbed percutaneously. Following application to the skin behind the ear, circulating plasma levels are detected within 4 hours with peak levels being obtained, on average, within 24 hours. The average plasma concentration produced is 87 pg/ml for free scopolamine and 354 pg/ml for total scopolamine (free + conjugates).
Distribution: The distribution of scopolamine is not well characterized. It crosses the placenta and the blood brain barrier and may be reversibly bound to plasma proteins.
Metabolism: Although not well characterized, scopolamine is extensively metabolized and conjugated with less than 5% of the total dose appearing unchanged in the urine.
Elimination: The exact elimination pattern of scopolamine has not been determined. Following patch removal, plasma levels decline in a log linear fashion with an observed half-life of 9.5 hours. Less than 10% of the total dose is excreted in the urine as parent and metabolites over 108 hours.
Clinical Results: In 195 adult subjects of different racial origins who participated in clinical efficacy studies at sea or in a controlled motion enviornment, there was a 75% reduction in the incidence of motion-induced nausea and vomiting3. Transderm Scop provided significantly greater protection than that obtained with oral dimenhydrinate.
In two pivotal clinical efficacy studies in 391 adult female patients undergoing cesarean section or gynecological surgery with anesthesia and opiate analgesia, 66% of those treated with Transderm Scop (compared to only 46% of those receiving placebo) reported no retching/vomiting within the 24-hour period following administration of anesthesia/opiate analgesia. When the need for additional antiemetic medication was assessed during the same period, there was no need for medication in 76% of patients treated with Transderm Scop as compared to 59% of placebo-treated patients5,6.
Transderm Scop is indicated in adults for prevention of nausea and vomiting associated with motion sickness and recovery from anesthesia and surgery. The patch should be applied only to skin in the postauricular area.
Transderm Scop is contraindicated in persons who are hypersensitive to the drug scopolamine or to other belladonna alkaloids, or to any ingredient or component in the formulation or delivery system, or in patients with angle-closure (narrow angle) glaucoma.
Glaucoma therapy in patients with chronic open-angle (wide-angle) glaucoma should be monitored and may need to be adjusted during Transderm Scop use, as the mydriatic effect of scopolamine may cause an increase in intraocular pressure. Transderm Scop should not be used in children and should be used with caution in the elderly. See PRECAUTIONS. Since drowsiness, disorientation, and confusion may occur with the use of scopolamine, patients should be warned of the possibility and cautioned against engaging in activities that require mental alertness, such as driving a motor vehicle or operating dangerous machinery. Rarely, idiosyncratic reactions may occur with ordinary therapeutic doses of scopolamine. The most serious of these that have been reported are: acute toxic psychosis, including confusion, agitation, rambling speech, hallucinations, paranoid behaviors, and delusions.
Scopolamine should be used with caution in patients with pyloric obstruction, or urinary bladder neck obstruction. Caution should be exercised when administering an antiemetic or antimuscarinic drug to patients suspected of having intestinal obstruction.
Transderm Scop should be used with special caution in the elderly or in individuals with impaired metabolic, liver, or kidney functions, because of the increased likelihood of CNS effects.
Caution should be exercised in patients with a history of seizure or psychosis, since scopolamine can potentially aggravate both disorders.
Since scopolamine can cause temporary dilation of the pupils and blurred vision if it comes in contact with the eyes, patients should be strongly advised to wash their hands thoroughly with soap and water immediately after handling the patch. In addition, it is important that used patches be disposed of properly to avoid contact with children or pets.